Geri®
A Revolutionary Incubator for Enhanced Fertility Outcomes


10
million embryos monitored
26 %
*clinical pregnancy rate increase
12 %
*live birth rate increase
Award-Winning Incubator with Integrated Continuous Embryo Monitoring System
The incubator is critical to an embryo’s survival while it is being cultured outside the uterus. Geri® is a compact, modular benchtop incubator that integrates time-lapse imaging to capture key developmental stages of embryo growth. Designed to provide individualised and undisturbed incubation, Geri® provides stable culture conditions in an optimal environment.
Focussing on What Matters:
the Embryo
Our benchtop incubator is designed to provide individualised, stable culture conditions that help create an optimal environment to improve embryo viability and quality.
Undisturbed Incubation
Fail-Safe Mechanisms
Real-Time Monitoring of Incubation Conditions
Improved Lab Efficiency
Integrated Embryo Monitoring
Modular Software Offer

Creating Optimal Culture Conditions
Changes in environmental variables can significantly impact media efficacy and embryo development.
Geri® is designed to minimise disruptions to the embryo’s environment, reducing stress from lid openings while supporting gamete function and embryo development
Six Individual Chambers
Disruption Free Analysis
- State-of-the-art integrated continuous embryo monitoring system.
- Individual microscope with high-resolution camera in each chamber for reduced camera movement.
Light
- A long-wavelength light source (550-650nm) in the cameras reduces light-induced damage to vulnerable, early-developing embryos.
- Reduced total energy output compared to traditional microscopy.
Temperature
- Double heating elements in each chamber ensure temperature stability.
- Rapid recovery of user set point within 60 seconds of closing the lid.
- 4 temperature sensors accurately detect abnormal conditions.
- Audible temperature alarm and option for external alarm connection.
- External temperature probe port.
Gas
- Regulator maintains consistent gas flow.
- Purge functionality for rapid recovery of user-defined values within 3 minutes.
- A CO2 sensor in each chamber detects abnormal gas conditions.
- Independent gas lines for each chamber.
- External CO2 probe port.
Humidity
- Aims to minimise increases in osmolality levels that can impact embryo development.
- Option to choose humidified or dry chamber based on customer experience.
- Humidity alarm can be enabled for each chamber.
- Humidity is continuously tracked by independent sensors and can be monitored on the incubator’s parameter screen.
The Effect of Humidified Incubation in Geri®
In vivo culture requires humid conditions. Geri® offers an optional humidified environment in each chamber, enhancing embryo development and potentially improving reproductive outcomes.
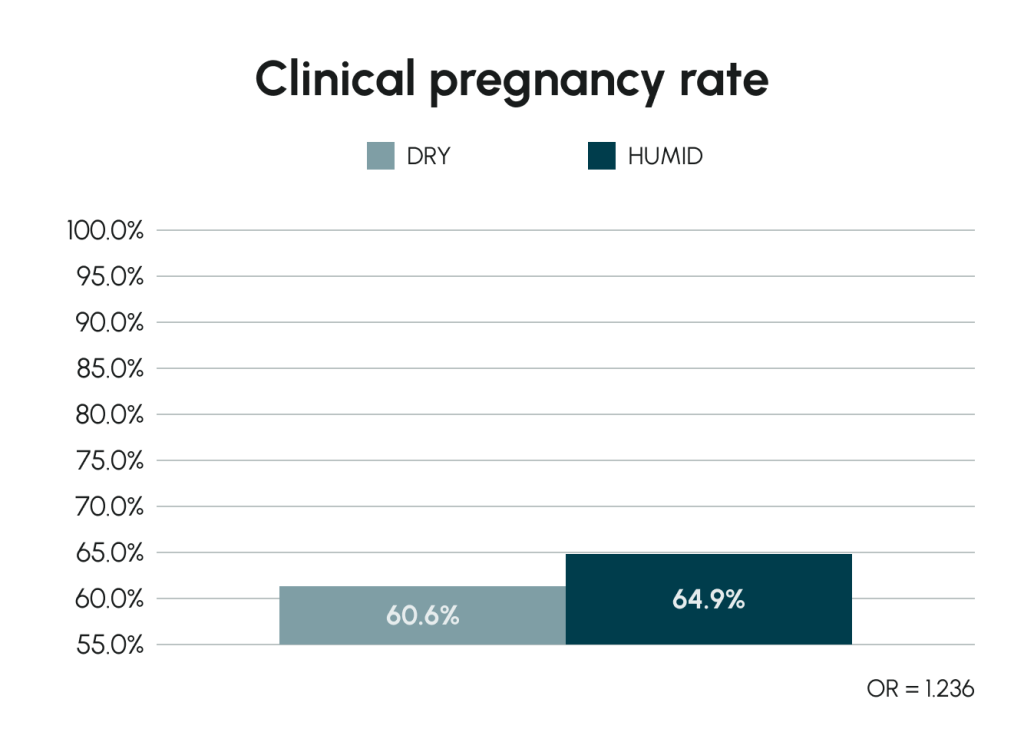
Valera et al (2022) Human Reproduction 37(9):1980–1993
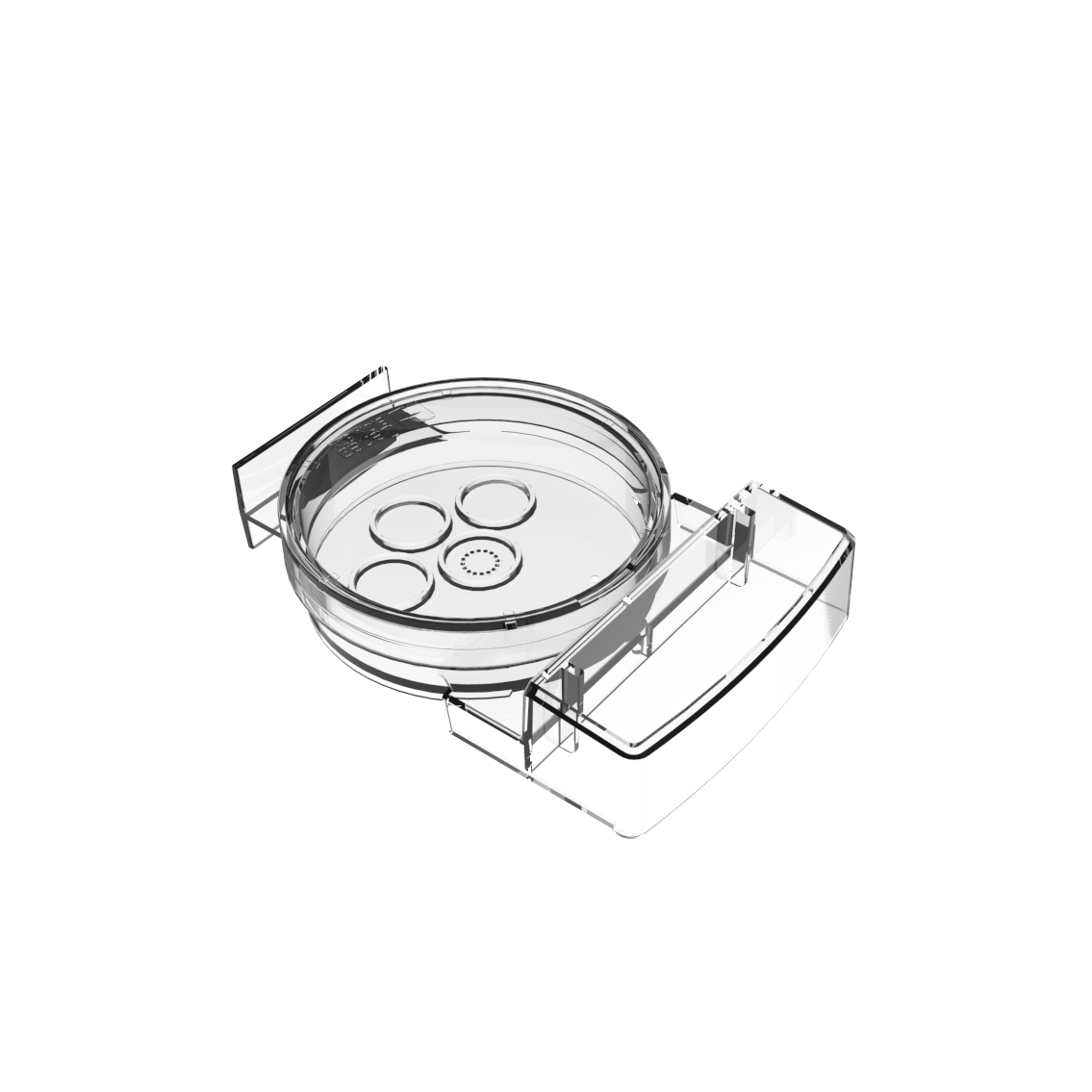
Smart Innovation: Geri® Dish
The Geri® dish has 16 microwells for individual embryo tracking whilst at the same time shared media allows for group culture, which may be beneficial for improved embryo development.
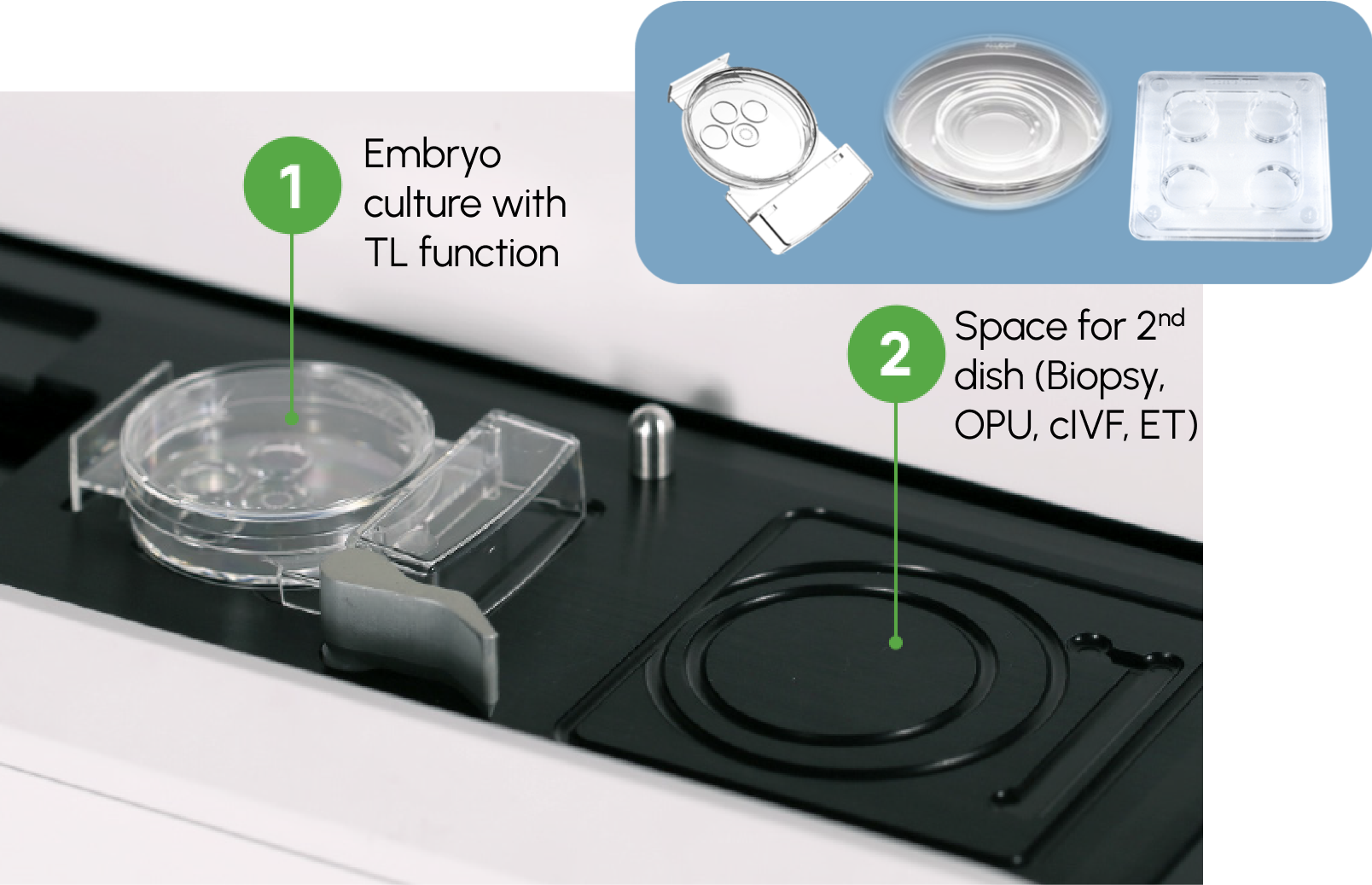
Geri®: Personalised Incubation
All of a patient’s dishes are cultured in Geri®
Reducing variability in gas, temperature, and pH conditions. There is no need for an extra incubator, eliminating additional costs for electricity, gas, and maintenance.

Get the most out of Geri® with Geri Connect & Assess®
Maximise Geri®’s efficiency with the advanced Geri Connect & Assess® system, which offers remote in-lab control to enhance workflow and laboratory operations. This system enables secure, confidential patient interactions both during consultations and via home access. Seamlessly share and export data as videos and images through its user-friendly interface.
Support Documents
Find the latest Instructions for Use (IFUs) for our products, including guidelines for safe and effective operation.







