Understanding the Stages of Human IVF Treatment
In vitro fertilisation (IVF) is a complex and multi-step process designed to help individuals and couples overcome infertility and achieve pregnancy. This blog post will provide an overview of the different stages of IVF treatment, highlighting key products and technologies offered by Genea Biomedx for each stage, including oocyte collection, fertilisation, embryo culture, cryopreservation and transfer, as well as steps related to managing key aspects of an IVF cycle safety.
1. Before IVF at the clinic: Hormonal Ovarian Stimulation
Ovarian stimulation before the steps conducted in the laboratory is a critical component of IVF treatment. Under natural conditions woman’s ovaries usually release one mature egg per menstrual cycle, but to increase the likelihood of successful fertilisation and embryo development, hormonal ovarian stimulation by targeted medication stimulates ovaries to produce several eggs simultaneously.
The process of stimulation and the overall IVF cycle starts with initial consultation with fertility specialist including baseline testing via blood samples and ultrasounds, to assess patients’ ovarian reserve and overall health. Ovarian stimulation protocols are tailored to the patient’s specific needs by their treating doctor, differing mainly by their use of either Gonadotropin-Releasing Hormone (GnRH) Agonists or Antagonists, resulting in faster or slower suppression of natural hormone production before Follicle-Stimulating Hormone (FSH), Luteinizing Hormone (LH) and human chorionic gonadotropin (hCG) injections.
GnRH Agonists and Antagonists at the start of the treatment prevent premature ovulation by suppressing body’s natural hormonal signals, FSH stimulates ovaries to produce multiple follicles, each potentially containing an egg, LH supports maturation of growing eggs and hCG induces final maturation of the eggs. All these medications are tightly controlled prescription medications manufactured by specialised pharmaceutical companies rather than medical device companies.
After an appropriate protocol has been established and natural hormone production suppressed, patients self-administer daily injections of FSH and LH for about 10-14 days. Throughout this phase, follicle development and hormone levels are monitored by blood tests and ultrasounds. Once the follicles reach the desired size, a trigger shot of hCG or GnRH agonist is administered to induce final maturation, followed by the next step in the overall IVF process, oocyte collection.
Genea Biomedx does not offer hormonal stimulation medications.
2. Gamete Retrieval: Egg and Sperm Collection
Once the follicles have matured, a minor surgical procedure for oocyte retrieval (egg collection) is performed. This involves using a thin needle, guided by transvaginal ultrasound, aspirating eggs from follicles. The procedure is typically done under sedation or anaesthesia in a day surgery, lasting usually less than 30 minutes. The aspirated solution is searched for the presence of eggs, which are transferred into the lab in dishes filled with specialised solution.
On the same day, the male partner or donor procures a semen sample, which is processed in the lab by washing and selecting the most motile sperm for fertilisation.
Key IVF products used with eggs include specialised oocyte aspiration buffer assisting in follicle flushing and oocyte handling media maintaining the viability of eggs during the subsequent handling. Sperm handling media or buffer as well as sperm wash solutions are used to wash and prepare the sperm for fertilisation.
Key products by Genea Biomedx that can be used for these steps include oocyte aspiration media (ORB), sperm media (SPM), sperm buffer (SPB) and sperm washing kit (SWG).
3. Fertilisation
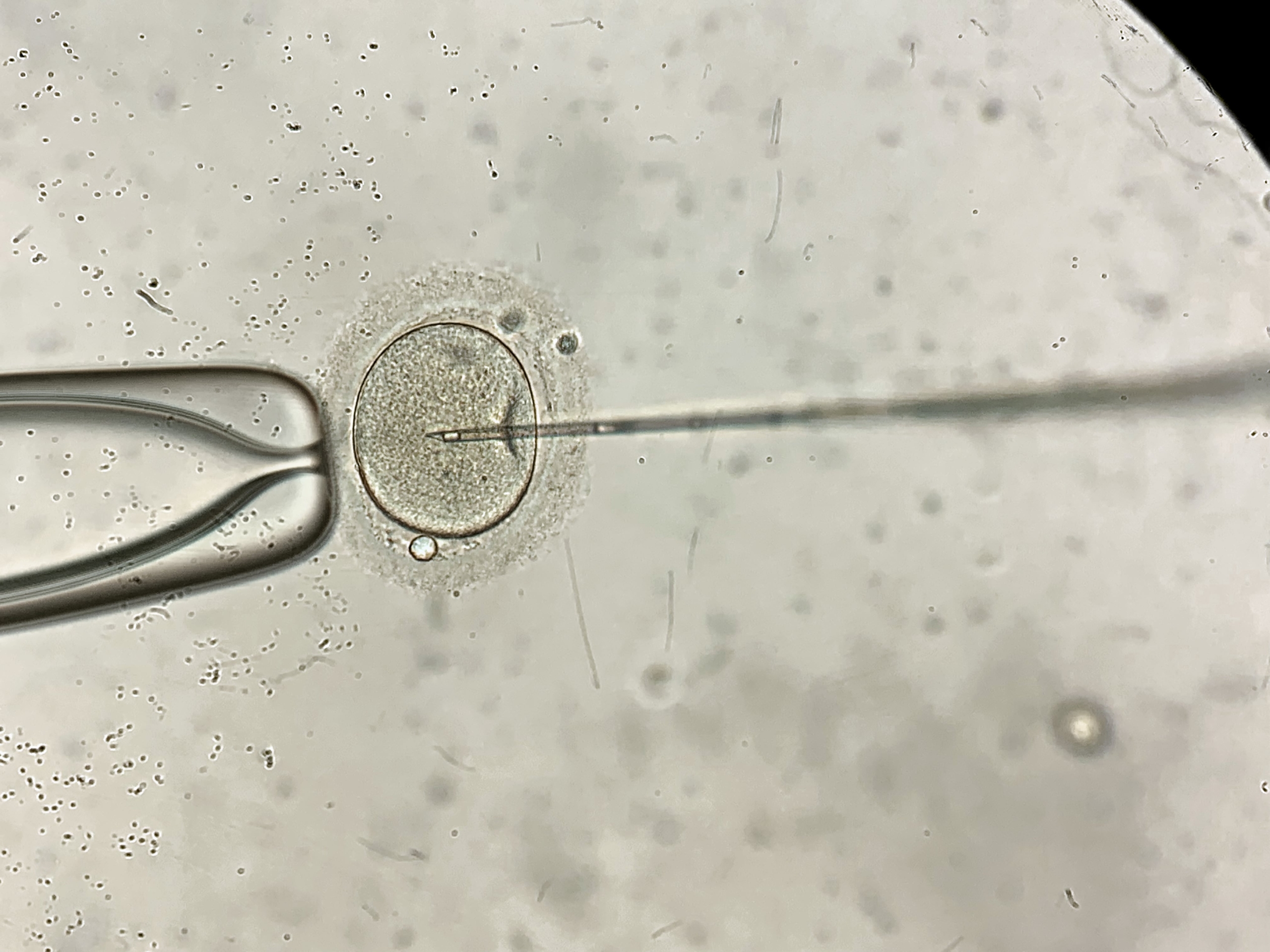
The collected eggs and prepared sperm are combined in a laboratory setting to facilitate fertilisation. This can be done through conventional insemination, where sperm is added to the eggs on a fertilisation dish and allowed to fertilise naturally, or through intracytoplasmic sperm injection (ICSI), where a single sperm is directly injected into an egg using specialised micropipettes and injection apparatuses set up on an inverted microscope system.
4. Embryo Culture
After fertilisation, the embryos are cultured in laboratory in an incubator with controlled temperature (+37°C) and gas environment (5% CO2 and either ambient 21% or reduced 5% O2) for up to 3, 5 or 6 days until they have reached cleavage or blastocysts stages. During this time, the embryos are monitored for development either by periodically removing them from a standard incubator and reviewing them under a microscope, or if using a timelapse incubator, by reviewing their development from a continuous live video recording.
Key products by Genea Biomedx that can be used for these steps include sequential embryo culture solutions cleavage medium (CLM) and blastocysts medium (BLM), continuous culture medium (ONE) and timelapse incubator and software (GERI and GCA).
5. Embryo Selection, Cryopreservation and Transfer
Once the embryos have reached the appropriate stage of development, best-quality embryos are selected for transfer into the uterus either immediately, or for cryopreservation to be transferred later. The number of embryos transferred or cryopreserved depends on various factors, including the number and quality of embryos available.
Final embryo selection is done by experienced embryologists, who however may use various supporting tools that can standardise and categorise embryos based on their appearance and developmental history.
Embryo transfer is conducted by depositing embryo transvaginally through a fine catheter filled with suitable solution in a minor procedure lasting only minutes.
Embryo cryopreservation is conducted either by slow freezing, or these days predominantly by vitrification, a rapid freezing technique that prevents ice crystal formation. In vitrification embryos are moved through solutions with increased levels of cryoprotectants over a period of up to 15 minutes, loaded into thin straws and plunged immediately to liquid nitrogen at -196 C, rendering them into a ‘glass-like’ stage. Slow freezing involves likewise moving embryos through cryoprotectant solutions, followed by cooling in a stepwise manner over a period of hours before plunging them into liquid nitrogen for storage.
Key products by Genea Biomedx that can be used for these steps include a third-party Eeva Test for embryo assessment, vitrification and warming sets (VIT-01 and WRM-01) and vitrification buffer (VBS). Geri system can also be used with several third party embryo assessment programs.

7. Managing clinic workflow
Throughout the IVF process, strict protocols are followed to ensure ideal workflows, efficiencies and safety of the procedures. An important aspect of this is the correct identification and handling of gametes and embryos to ensure integrity of the process and to avoid any mix-ups or mistakes. IVF witnessing can be conducted by manual double-checking and verifying the identity of samples and correctness of each step by two different embryologists, or alternatively, with the help of digital witnessing systems.
Key features and benefits of electronic systems include automated identification of barcode or Radio Frequency Identification (RFID) tags to automatically identify and track labelled samples, container and documents, with each patient assigned a unique identifier. The systems monitor handling and transfer of gametes and embryos continuously, providing real-time alerts if there is a mismatch or if an incorrect procedure is about to occur, helping to prevent errors before they happen. In addition, the systems record every step of the process, providing a detailed log of all actions taken. This helps clinics to comply with regulatory requirements and maintain high standards of quality control, overall improving the accuracy, safety, and efficiency of IVF treatments and providing peace of mind for both patients and clinicians.
Conclusions:
IVF is a highly intricate process that requires meticulous attention to detail and the use of specialised products and technologies at each stage. From ovarian stimulation to embryo transfer and cryopreservation, each step is crucial for the success of the treatment. By understanding the stages of IVF and the products involved, we can better appreciate the complexity and precision required to achieve a successful outcome.
