Gavi®
World´s first automated vitrification instrument


100 %
*Human blastocyst recovery rate
5,3 %
**Increase in clinical pregnancy rate
For Standardised Vitrification
with High-Quality Results
Vitrification is the most up-to-date and effective method of freezing oocytes and embryos, regardless of stage. Speak to embryologists about manual vitrification, and they will readily agree that the process can be tedious and time-consuming. There is a long learning curve, skills must be maintained, and intense focus is required of the embryologist.
Gavi®, the world’s first automated vitrification instrument, was designed by embryologists seeking better control over the variables involved in manual vitrification—temperature, embryo handling, cryoprotectant concentrations, and balancing equilibration/vitrification timing. Their aim was stage-specific, controlled vitrification performed in a standardised way every single time—and they succeeded.
Explore Gavi®:
Precision and Consistency in Embryo Vitrification and Storage
Created after four years of intensive development, Gavi® is the first technology to automate the critical equilibration step, standardising the complex vitrification process.
Gavi® takes a completely new approach to vitrification, helping to achieve consistently high-quality results every time.
Consistent and Reproducible Process
Unique Gavi® Pod
Precise Equilibration Process
High-Quality Results
Controlled, Closed-System Environment
Short Learning Curve

Gavi® Helps You Achieve Optimal Vitrification Conditions.
There are many critical variables within the vitrification process that can impact embryo survival post-thawing – from achieving the right balance between the cryoprotectant concentrations and flow rates to reducing manual handling.
Automated Solution Dispensing
Gavi® Pod Design
Control of Exposure
Temperature Control
Reduces the Risk of Cross-Contamination

The Gavi® Family
Gavi® comes with a range of supplied accessories, including:

Gavi® Operating Tray
Holds the Gavi® Medium Cartidge, Cassete and Tip & Seal Catridge.

Gavi® LN2 Bucket
Holds liquid nitrogen for the final stage of the vitrification process.

Gavi® Tweezers
For a secure grasp of the Gavi® Cassette when dunked into Gavi® LN2 Bucket.
Consumables used with Gavi®

Gavi® Tip & Seal Cartridge
Holds the disposable pipette tip and Pod lid seal.
Gavi® Medium Cartridge
Contains the Gems® vitrification solutions.

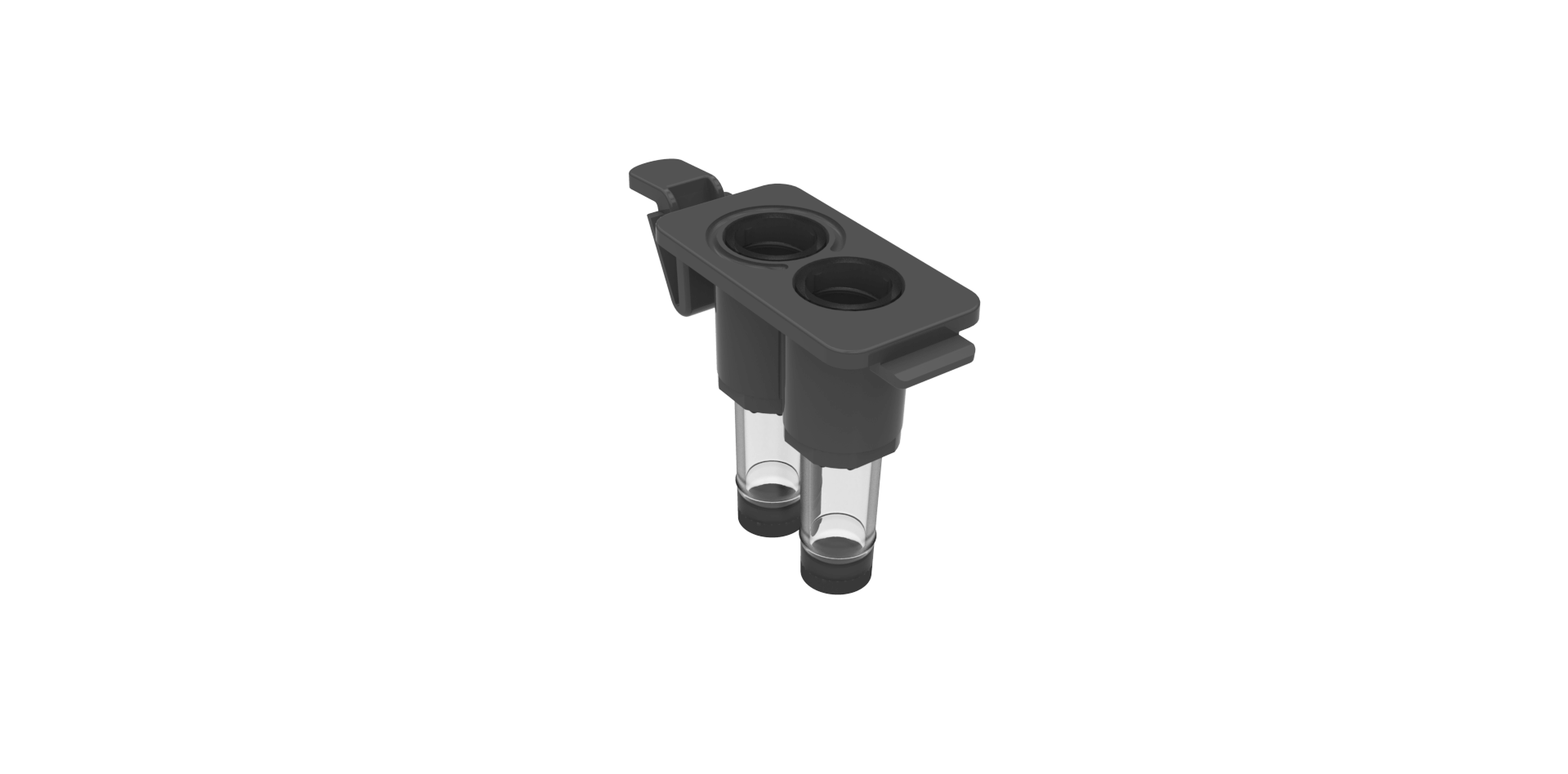
Gavi® Cassette
Holds up to four Pods at once, allowing you to vitrify four blastocysts or eight oocyte/cleavage stage embryos simultaneously
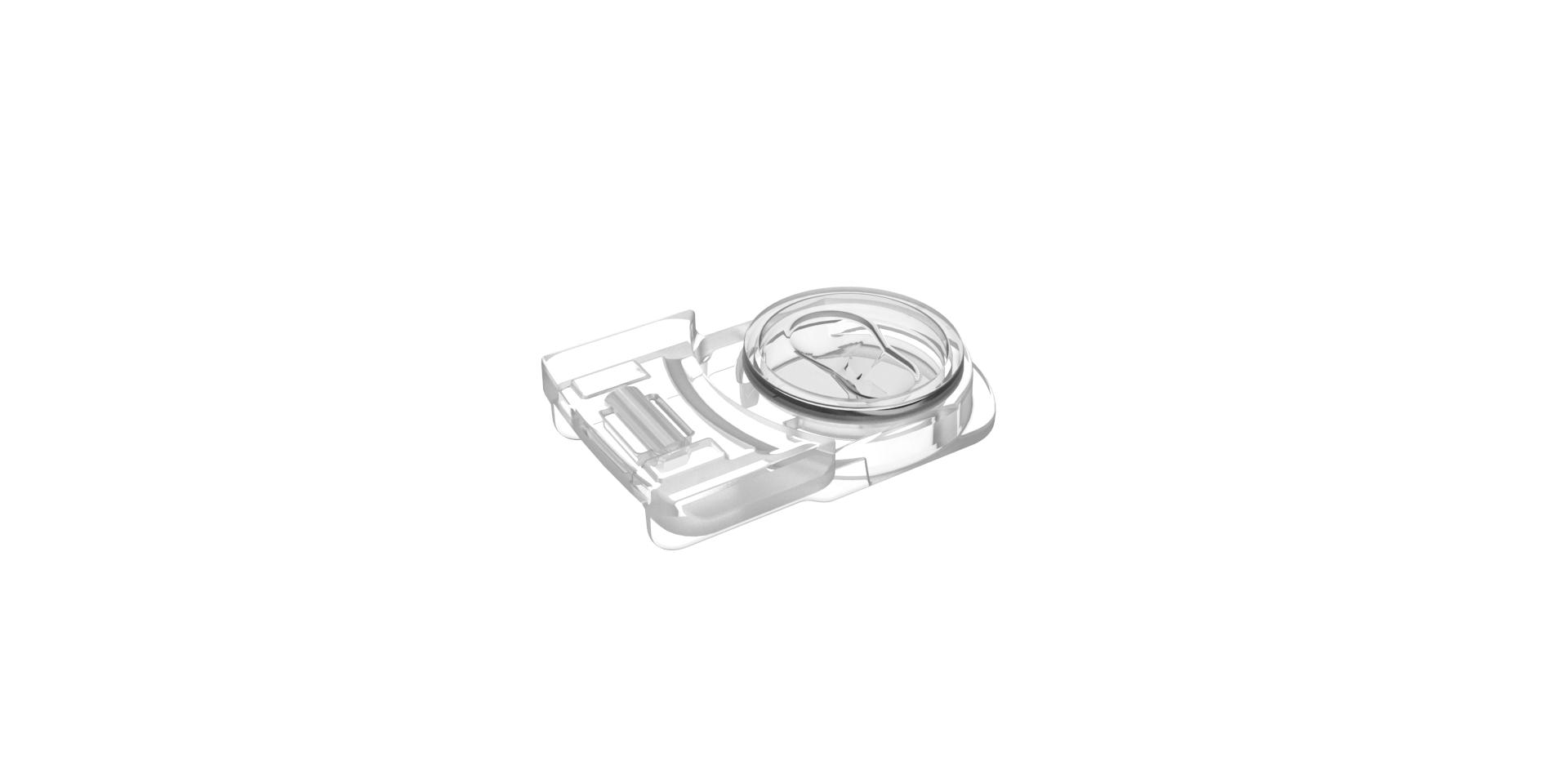
Gavi® Pod
The Gavi Pod is a container with the capacity to hold two oocytes or zygote/cleavage stage embryos or one blastocyst stage embryo during the vitrification, storage and warming processes.
Accessories used with Gavi®

Gavi® Working station
Holds the Gavi® cassette within LN2 to facilitate the removal of individual Pods for thawing.

Gavi® Storage Dividers
For use with cryogenic storage canisters, providing orderly organisation of Cassette and Pods by patient. Available in round or rectangular forms.
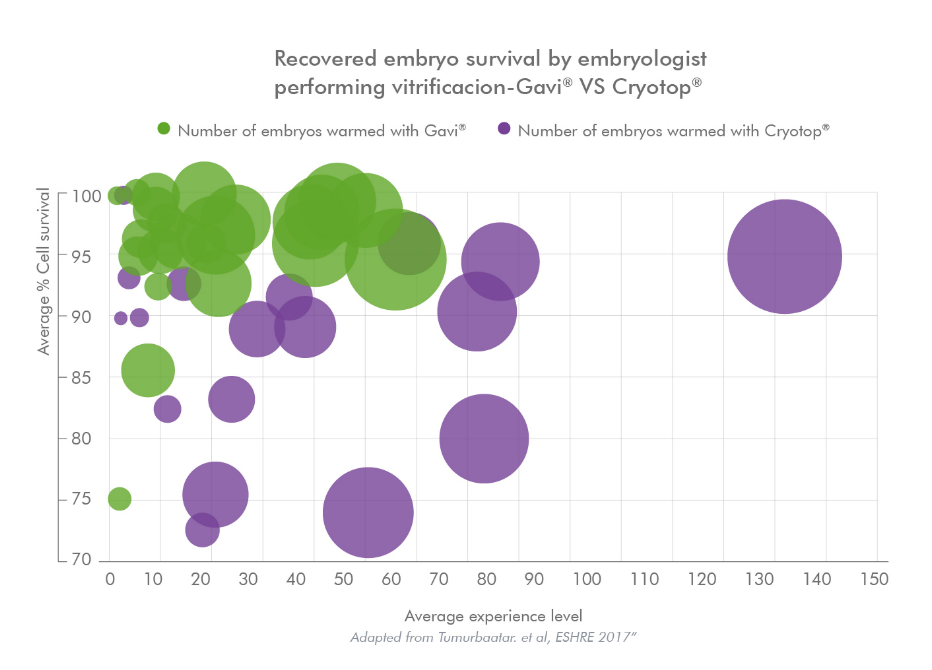
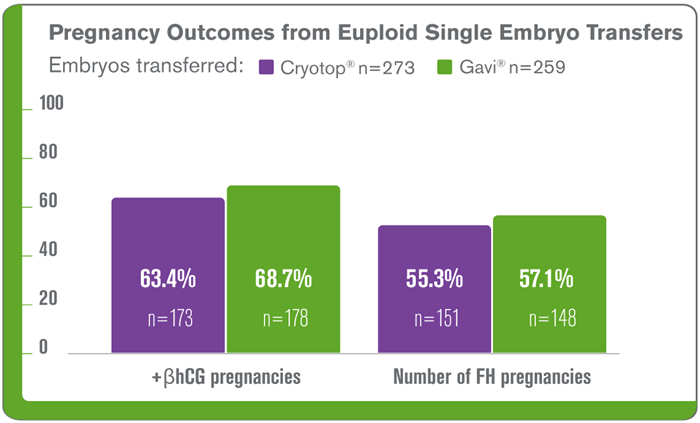
Greater Consistency of Results Compared to Manual Methods.
To evaluate the potential improvements from a training and standardisation perspective, survival results across freeze scientists for the early stages of implementation at all Genea labs of Gavi® (2015-2017) and Cryotop® (2009-2010) were retrospectively analysed.
When comparing blastocyst survival, Gavi® reduces the variability of results among embryologists of different experience levels with using particular device.
Gavi® requires reduced technical skill compared to manual vitrification methods, with shorter learning curves and a reduced time to achieve vitrification competency
Gavi® Clinical Outcomes:
The Evidence Lies in the Results
Any implementation period provides an opportunity to analyse data and so we looked back. It became obvious how easy Gavi® was to learn and how quickly embryologists were able to achieve benchmark results compared to the manual method.
When analysing the early-stage implementation data for the two techniques we found using Gavi® resulted in a reduction in the variability of results between embryologists and the period between basic vitrification competency and aspirational competency was reduced.
Alpha Scientists in Reproductive Medicine (2012). The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod. Biomed. Online 25: 146-167.
Support Documents
Find the latest Instructions for Use (IFUs) for our products, including guidelines for safe and effective operation.