Gems®
Your Complete Range of IVF and Vitrification Media


30 +
Development history
26 %
Clinical pregnancy rate increase*
12 %
Live birth rate increase*
10.000
IVF Babies born
Designed by Embryologists Who Use It
Genea Biomedx’s dedicated embryologists and andrologists have worked passionately in assisted reproductive technology for decades. We understand that high-quality culture media is essential for achieving the best possible outcomes for patients. That is why we have developed, produced, and used our own media formulations since 1991. Gems®, our third-generation culture media suite, has been in clinical use since 2013 and is exported globally.

Gems®: The Third Generation of Our Culture Media Suite
From gamete analysis to vitrification.
Optimised for Every Stage
Robust and Effective Media
Rooted in Heritage
Established Safety and Efficacy
Consistently High-Quality Results, Batch After Batch
User-Friendly
Optimised for Every Step
Gems® adapts to your laboratory practices and workload demands with a complete range of IVF and vitrification media , with most media in the suite available in two bottle sizes (20ml and 50ml).
Gems Categories
Gamete Handling & Preparation

Oocyte Retrieval Buffer 50 mL
Conceived to reduce stress on the occytes during their retrieval from ovarian follicles.

Sperm Medium 50 mL
Used to wash and resuspend sperm for the insemination step, in IUI, IVF or in diganostic washing, optimised for usage in a 6% CO2

Sperm Wash Gradient Set
Used to separate sperm from seminal plasma as well as separating highly motile sperm in preparation for insemination.

Sperm Buffer 50 mL
Used to wash and resuspend sperm for the insemination step in intrauterine insemination (IUI), IVF or in diagnostic washing
Growth Media
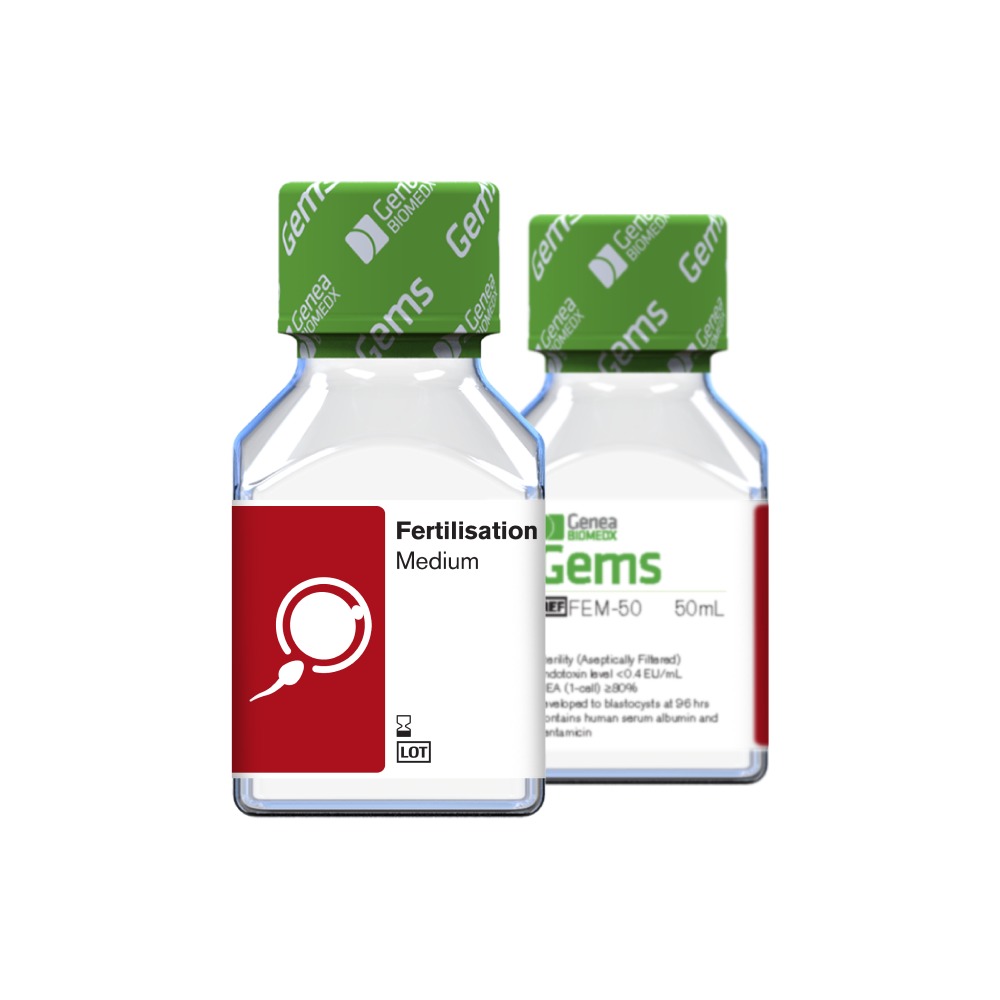
Fertilisation Medium 50 mL
Used to provide a suitable environment for both oocytes and sperm, to promote optimal fertilisation rates. Supplemented with human serum albumin (5 mg/mL) and gentamicin (0.01 mg/mL)

Cleavage Medium 50 mL
Higher EDTA and concentration of pyruvate, lactate and non-essential amino acids, to support the embryo to reach the cleavage stage

Blastocyst Medium 50 mL
Higher concentration of glucose and essential amino acids, to support the embryo development from cleavage to the blastocyst stage.

Geri® Medium 50 mL
Ready-to-use solution to support extended culture with uninterrupted incubation up to blastocyst stage. Embryos retain their microenvironment for the entirety of in vitro culture.
Vitrification & Warming Solutions

Vitrification Set
Cryoprotectant solutions protect against cell damage (EG/DMSO/Trehalose). For the manual vitrification of human embryos.

Warming Set
For the warming of embryos vitrified using either the Vitrification Set or the Gavi® Medium Cartridge.
Geri® in combination with Geri® Medium
Provides stable culture conditions to support improved embryo quality.

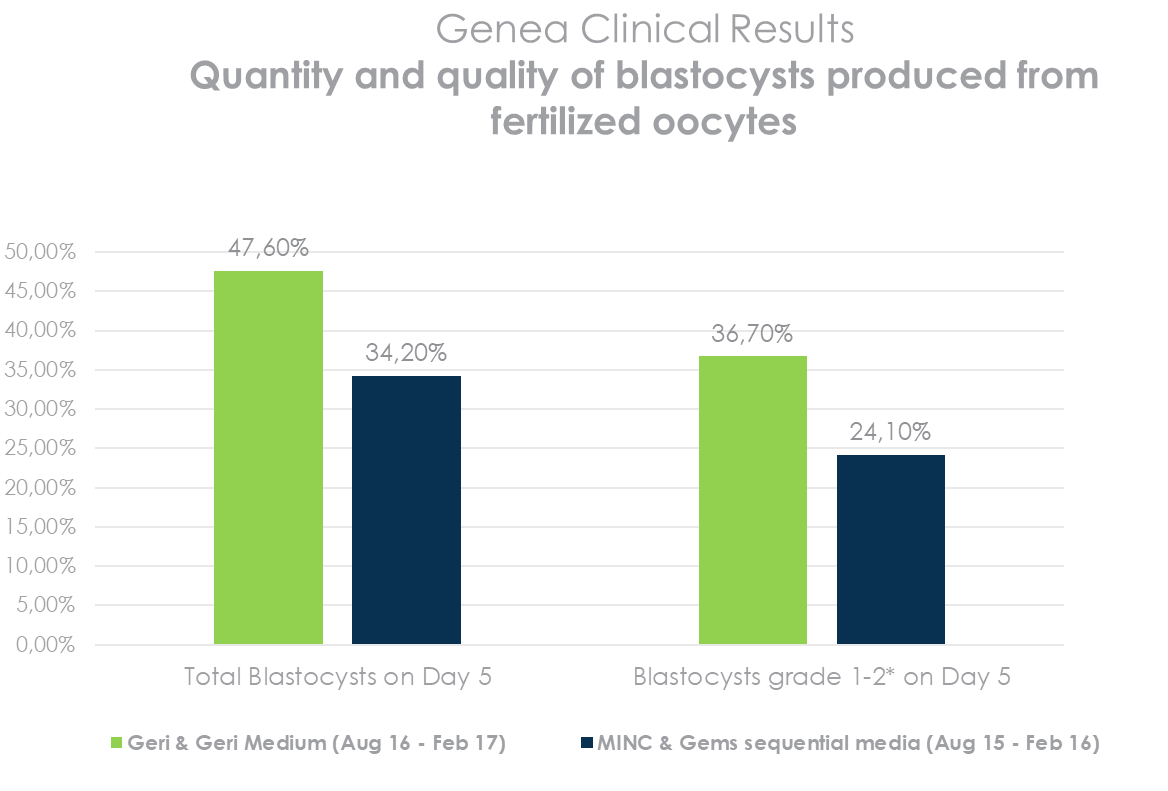
Increased Production of Higher-Quality Blastocysts*
*QRTV321_Human Embryo Culture using Geri Medium. Source: Table 4 – Summary of morphological assessments of embryos cultured in Geri Medium or Gems sequential media, utilising either the Geri or MINC incubator.
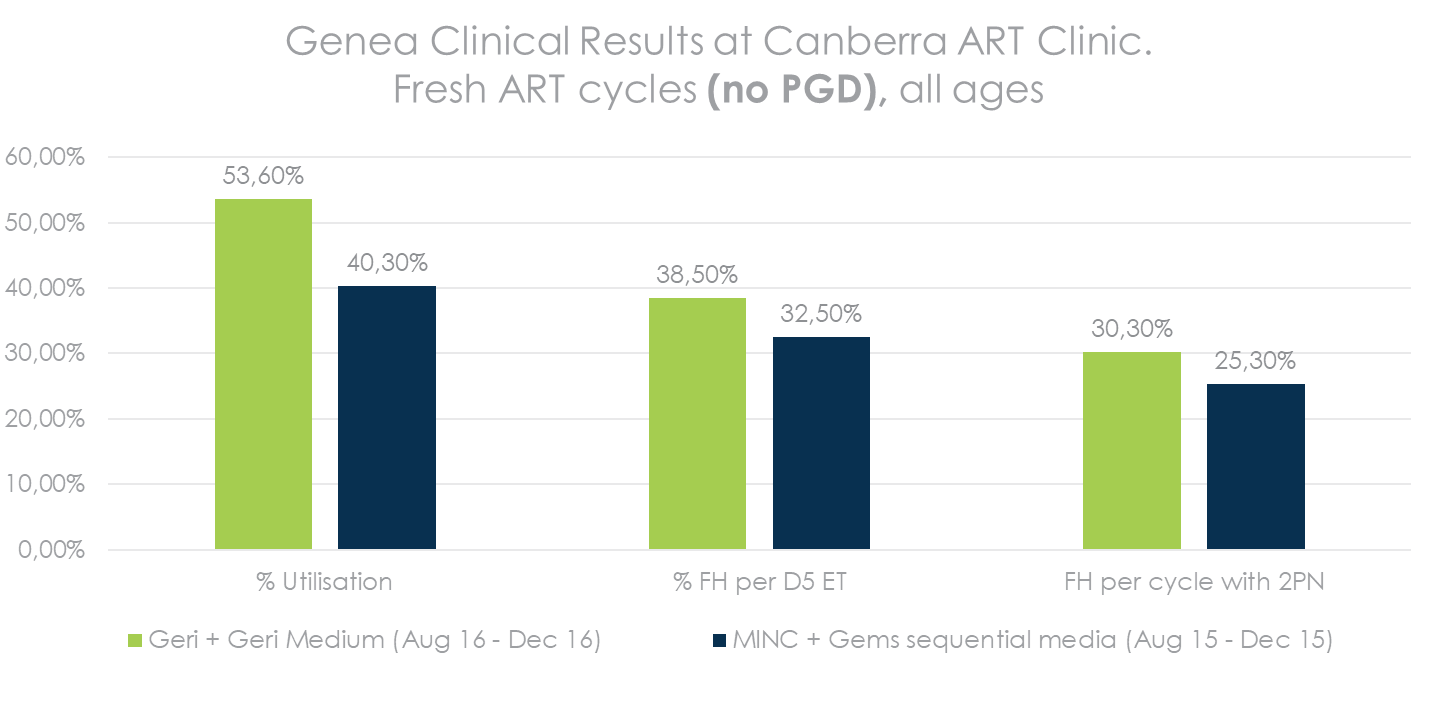
Increase in %FH per cycle with 2PN = more patients with a successful cycle
Higher Utilisation & Pregnancy Rate*
*QRTV321_Human Embryo Culture using Geri Medium. Source: Table 5 – Summary of pregnancy outcomes following embryo culture using each system
Support Documents
Find the latest Instructions for Use (IFUs) for our products, including guidelines for safe and effective operation.