Gidget®
Optimising Fertility Lab Efficiency with Innovative Tracking Technology


Easy-to-use Electronic Witnessing System
Gidget® is an easy-to-use electronic witnessing system designed to aid sample identification and tracking during ART procedures, while minimising the chance of human error. Not only is it an electronic witnessing system, but it also provides a customisable workflow management system that is configurable for any clinical lab.
Gidget facilitates the identification and traceability of IVF samples in accordance with EU directives and best-practice scientific guidelines for electronic witnessing.
A Modular, Easy-to-Use Solution for Every Lab
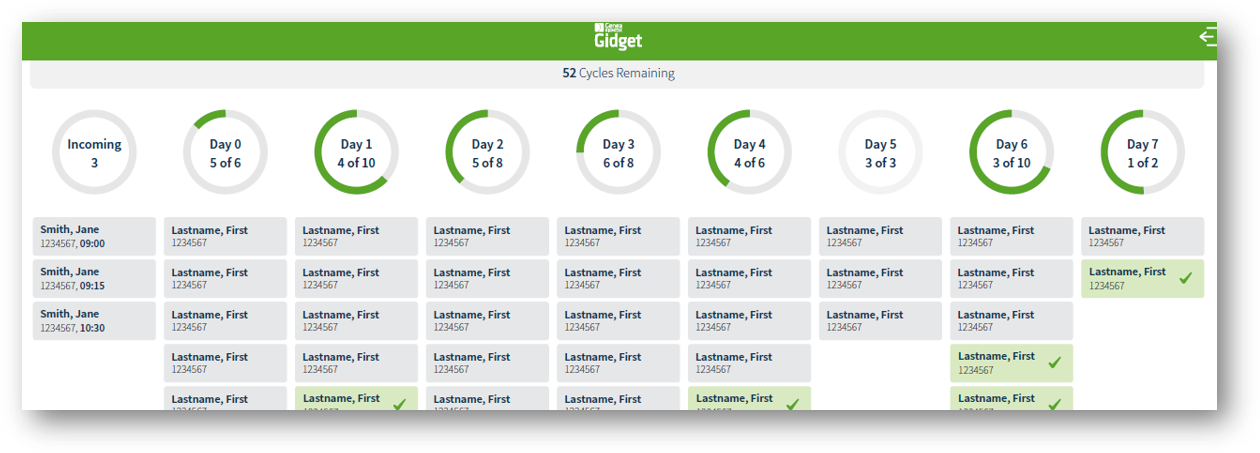
Gidget® is an electronic witnessing and workflow management system within the ART lab and clinic environment, enabling high workflow visibility, process and consumable traceability, and streamlined reporting.
Confidence
Efficiency
Versatile
Traceability
Patient Support
Cost-Effective

Designed To Adapt to Your Lab’s Existing Infrastructure and ART Procedures:
The low complexity of Gidget® allows for easy and fast integration into the lab. Gidget® communicates via Wi-Fi, and no modifications to the lab’s furnishings are required.
In addition, its modular approach enables the integration of new functionalities with minimal disruption to the lab.
Customised To Suit Individual Laboratory Needs:
Anywhere, Anytime:
Don’t Miss Anything:
Easy-To-Use Interface:
Everything at a Glance:
Handheld Scanner
Workflow
Management
In addition to electronic witnessing, Gidget® incorporates process scheduling tools to help manage task workflow and improve traceability in the laboratory.
Gidget® provides risk minimisation and change control for increased reliability and traceability in the ART lab, beyond witnessed processes, with exportable user traceability logs and streamlined reports that can be used for audits, inspections, and accreditation.
Real-Time Information & Monitoring
Workflow Customisation
Traceability & Reporting
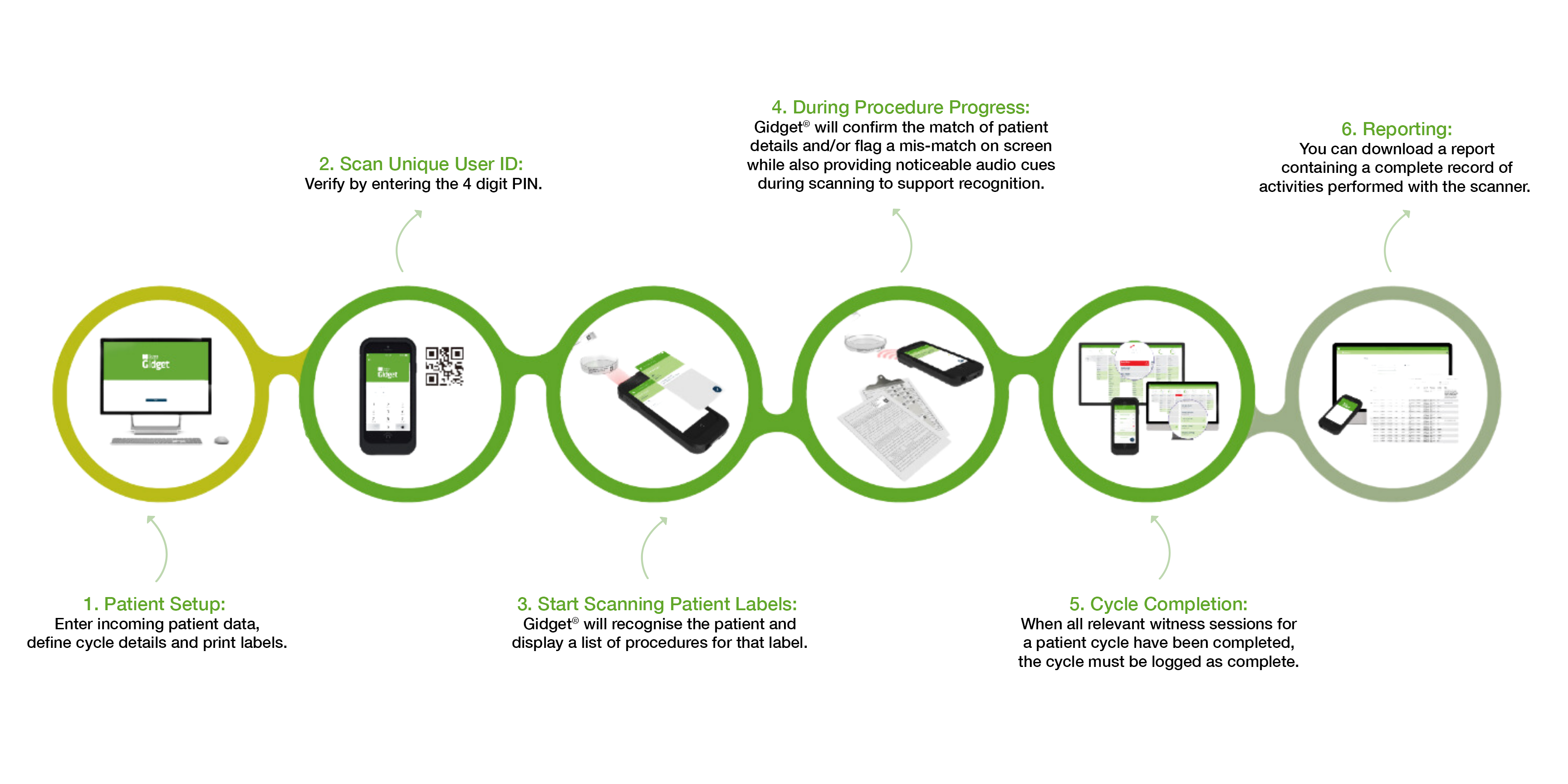
How Gidget® Works
Upon installation, Gidget® will be configured according to your clinic’s processes. Laboratory supervisors and administrators define workflow rules.
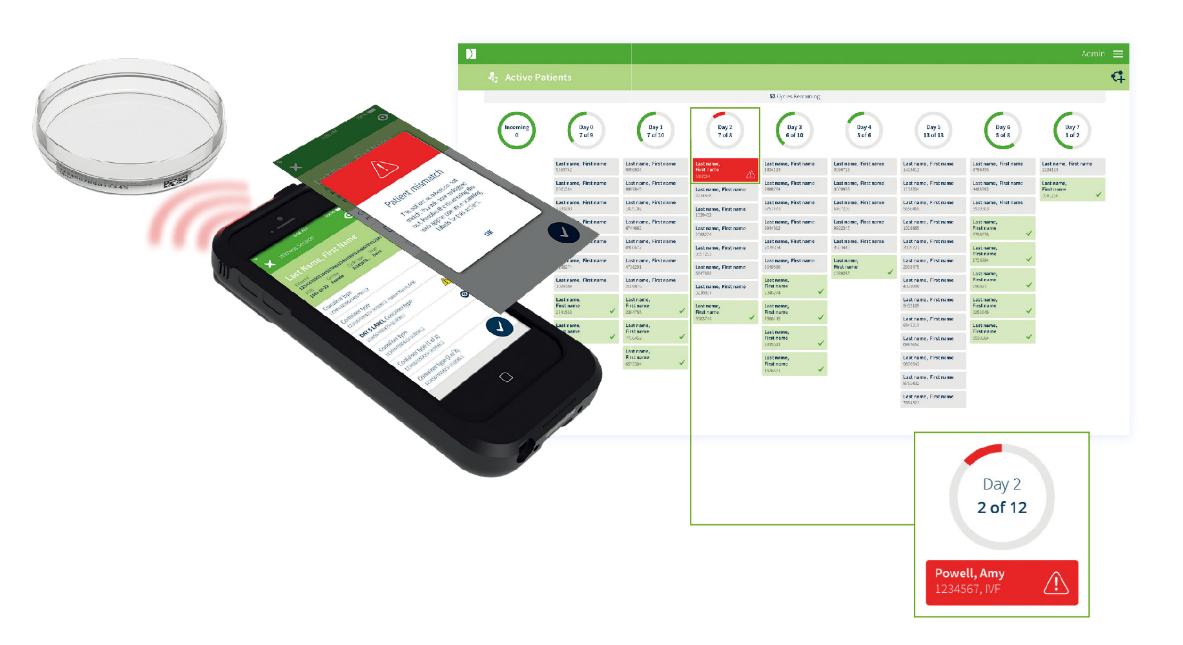
Electronic Witnessing
Two levels of critical mismatch errors are highlighted across devices as soon as they happen and require supervisor approval to proceed further aiding quality control.
- Patient mismatch error
- Sample mismatch error
Support Documents
Find the latest Instructions for Use (IFUs) for our products, including guidelines for safe and effective operation.